[New Year, New Weather!! D-innovation’s First Transfer-A API in 2024-Phloroglucinol/Phloroglucinol Trimethyl Ether]
[New Year, New Weather!! D-innovation’s First Transfer-A API in 2024-Phloroglucinol/Phloroglucinol Trimethyl Ether]
A good start to the New Year! ! On January 11, 2024, Center for Drug Evaluation (CDE) of National Medical Products Administration registered our product
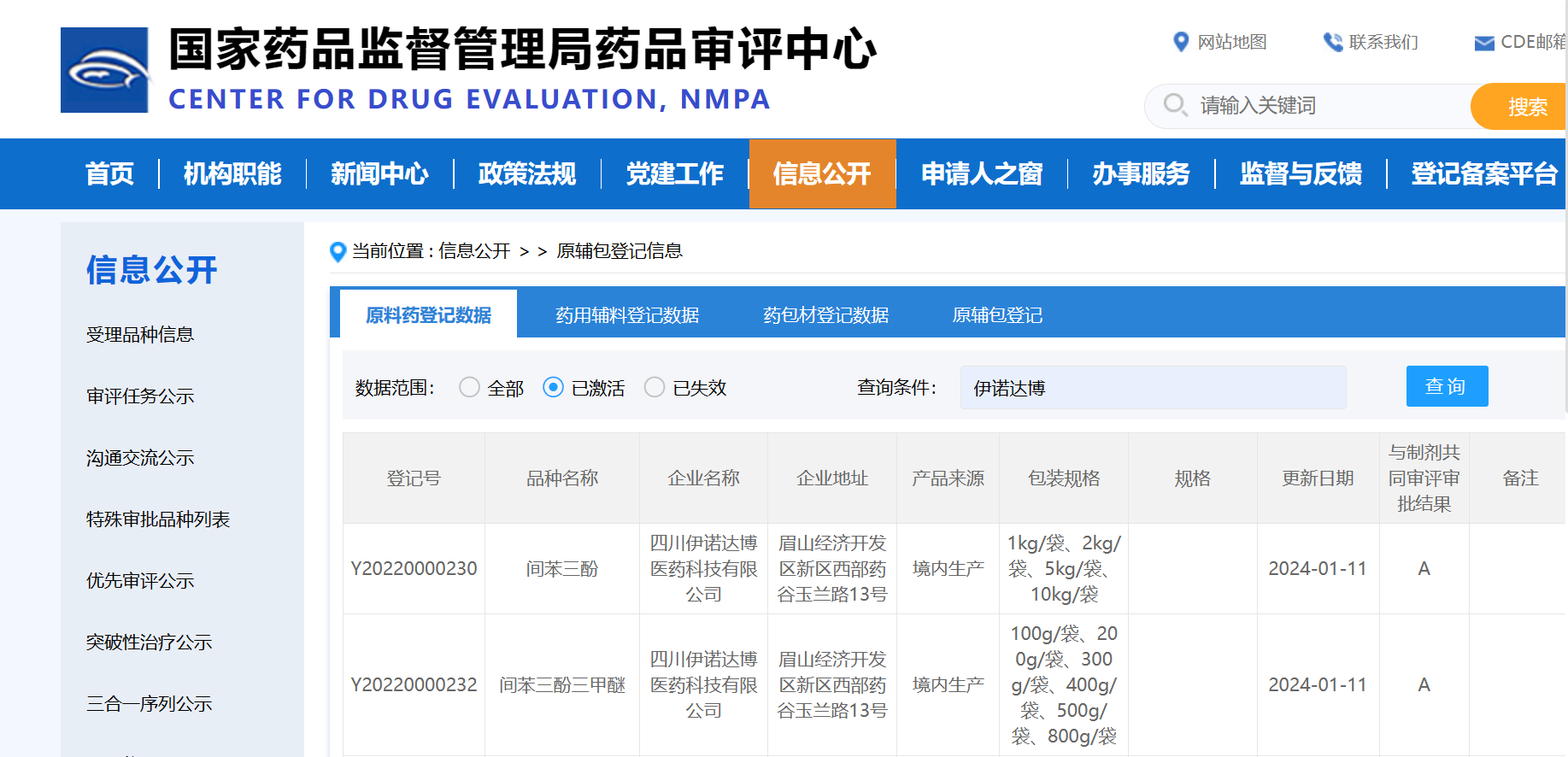
phloroglucinol/phloroglucinol trimethyl ether as status A!!!
Phloroglucinol/phloroglucinol trimethyl ether can directly act on the smooth muscle of gastrointestinal tract and urogenital tract, and is a myotropic non-atropine and non-papaverine pure smooth muscle spasmolytic. It can be used for acute spasmodic pain caused by digestive system and biliary tract dysfunction; acute spasmodic urethral, bladder, renal colic; gynecological spasmodic pain. It is characterized by no anticholinergic effect and little side effect on cardiovascular system.
Basic information:
name: phloroglucinol name: phloroglucinol trimethyl ether
Phloroglucinol 1,3,5-Trimethoxybenzene
CAS No.: 108-73-6 CAS No.: 621-23-8
Molecular formula: C6H6O3 Molecular formula: C9H12O3
Molecular weight: 126.11 Molecular weight: 168.19
Appearance: White to light white crystalline powder Appearance: White crystalline powder
Melting range: 215 ℃ -220 ℃ Melting range: 50 ℃ -53 ℃
Approved Dosage Form:
1. Oral lyophilized tablets (80 mg/tablet)
2. Injection (4 mL: 40mg/tube)
3. Freeze-dried powder injection (40 mg/bottle)
PS: phloroglucinol/phloroglucinol trimethyl ether ratio of 1000:1
Project progress
Phloroglucinol, registration number: "Y20220000230", registration status: "A"
Phloroglucinol trimethyl ether, registration number: "Y20220000232", registration status: "A"
The core business of D-innovation
Related declaration service for generic APIs
R & D and large-scale production services of generic API intermediates
CMC Research Services for Innovative Drug Substances
CDMO services for innovative drug APIs and intermediates


